|
||
| PRESENTED BY THE ALLIANCE FOR PHARMACY COMPOUNDING | ||
| Axios Vitals | ||
| By Maya Goldman and Tina Reed · Jun 17, 2025 | ||
|
Good morning, Vitals readers! Today's newsletter is 940 words or a 3.5-minute read. Situational awareness: The Senate Finance Committee yesterday released its long-awaited health and tax provisions, with some controversial moves to get more savings from Medicaid, Peter Sullivan and Victoria Knight wrote for Axios Pro. |
||
| 1 big thing: NIH ruling is the latest blow to RFK Jr.'s agenda | ||
| By Tina Reed and Steph Solis | ||

|
||
|
Illustration: Annelise Capossela/Axios |
||
|
Hundreds of researchers who saw their NIH-funded studies halted by the Trump administration could begin working again soon after a federal judge ordered their funding restored yesterday. Why it matters: The ruling was the latest blow to HHS Secretary Robert F. Kennedy Jr.'s efforts to reshape the agency, including cutting funding for research and institutions that it says do not support the agency's mission, such as diversity, equity and inclusion studies.
Driving the news: NIH cut nearly $3.8 billion in grants to U.S. institutions, per estimates from the Association of American Medical Colleges.
The other side: HHS said it is exploring all legal options, including filing an appeal and moving to stay the order.
|
||
|
|
||
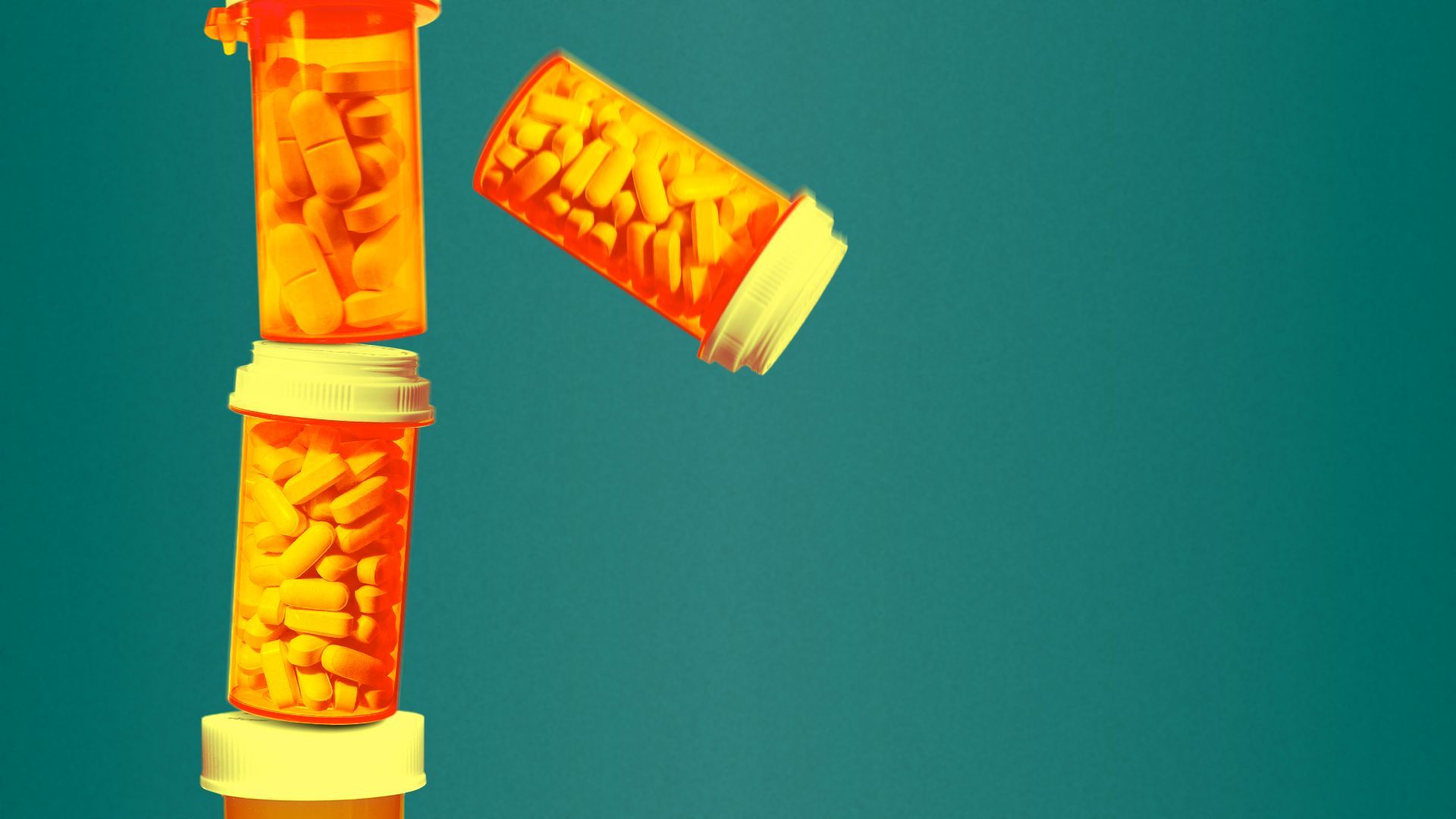
| 2. States agree to opioid settlement | ||
| By Maya Goldman | ||
|
||
|
Megan Robinson/Axios |
||
|
Attorneys general from 55 U.S. states and territories signed onto a $7.4 billion settlement with Purdue Pharma and the Sackler family for their role in the country's opioid epidemic, officials said Monday. Why it matters: The settlement, first announced in January, ends the Sackler family's control of Purdue Pharma and prohibits them from selling opioids in the country going forward, per state officials.
State of play: The funds will be distributed over 15 years, with the majority going out to states in the first three years, per the deal. The Sacklers will pay $1.5 billion in their first payment, and Purdue will pay about $900 million.
|
||
|
|
||
| 3. 23andMe's former CEO agrees to buy company | ||
| By Dan Primack | ||

|
||
|
Illustration: Lindsey Bailey/Axios |
||
|
Anne Wojcicki is poised to regain control of genetics testing company 23andMe, which she cofounded and led until earlier this year, after agreeing to put much of her personal fortune on the line. Why it matters: This is the same person that 23andMe customers originally trusted with their personal data, albeit under a much more opaque governance structure. Catch up quick: Regeneron Pharma in May won a bankruptcy auction for 23andMe's assets, bidding $265 million and beating out Wojcicki (who'd been trying to purchase the company since well before the Chapter 11 filing).
Look ahead: A court hearing to approve Wojcicki's bid is scheduled for today. The bottom line: Even if she clears all the legal hurdles, it remains unclear how Wojcicki plans to make 23andMe into a viable business. |
||
|
|
||
|
A MESSAGE FROM THE ALLIANCE FOR PHARMACY COMPOUNDING |
||
| 14 ways to cut red tape — and protect patient care | ||
|
|
||
|
Ineffective policies are limiting access to critical compounded drugs — patient-specific therapies prescribed when an FDA-approved drug is inappropriate or unavailable. What you need to know: Our Blueprint outlines 14 targeted reforms to protect patient care. |
||
| 4. FDA hasn't approved doc-favored sunscreen | ||
| By Carly Mallenbaum | ||

|
||
|
Illustration: Allie Carl/Axios |
||
|
Dermatologists had hoped that an active sunscreen ingredient popular in Europe would be available in the U.S. by summer — but the FDA still hasn't approved it. Why it matters: U.S. sunscreens lack some of the advanced UVA protection found in international brands, due to outdated FDA regulations, experts say. Catch up quick: Bemotrizinol, a sunscreen filter, seemed close to FDA approval late last year.
What we're hearing: Bemotrizinol — which has been available in sunscreens in other parts of the world for years — is photostable (doesn't break down once exposed to sunlight) and better at protecting against UVA rays than U.S. filters, dermatologist Henry Lim told Axios. Between the lines: International sunscreens sold in the U.S. — like Korean brands — are often modified or reformulated to meet U.S. regulations. What's next: "The soonest a decision will be made is 2026," Nazanin Saedi, a dermatologist and associate professor at Thomas Jefferson University, told Axios. "So do not delay in stocking up on your sunscreen for this summer!" |
||
|
|
||
| 5. Catch up quick | ||
|
❌ New guidelines for Veterans Affairs hospitals could allow workers to decline to treat patients based on personal characteristics not prohibited by anti-discrimination law, like political affiliation. (The Guardian) |

